The Art of Snow
Looking at snow up close will probably make you notice that
it is formed from thousands of little flakes which have stunningly complex
designs.
These snowflakes are actually ice crystals. They are formed
in the atmosphere, up in the clouds, and transform along their journey to Earth,
all thanks to different factors and forces that are involved in the process.
Let’s look at the facts on how snowflakes are formed, and
what atmospheric conditions are involved that contribute to the stunning
details we have come to know them for.
How is a Snowflake Built?
The designs of snowflakes are actually products of a
crystallization process that is controlled by the atmosphere.
Water vapor in the atmosphere latches onto a free-floating
speck of pollen or dust and acts as a nucleator. This means that from this
point, it can begin to add on (i.e. nucleate) more water molecules and mature
in size. When this process takes place at cold temperatures, water also freezes
and crystallizes.
Despite the many distinctive styles of snowflakes, their
crystallization occurs in the exact same shape: a hexagon. The reason for this
is related to how water behaves at the chemical level. At room temperature,
water molecules flow haphazardly around each other, forming and breaking bonds
endlessly.
However, when the temperature cools down, the water
molecules lose kinetic energy and form more stable bonds. By 0°C, they reorient
themselves into an energetically-efficient position, which happens to be a
rigid, hexagonal pattern. This is frozen water, or ice.
All snowflakes nucleate and crystallize this way. As more
water molecules nucleate to the infant snow crystal, they crystallize long arms
and branching tendrils, forming unique, artistic designs.
How these designs materialize is simply a matter of water availability
and temperature, a relationship that is best described in the Nakaya Diagram of
Snowflakes.
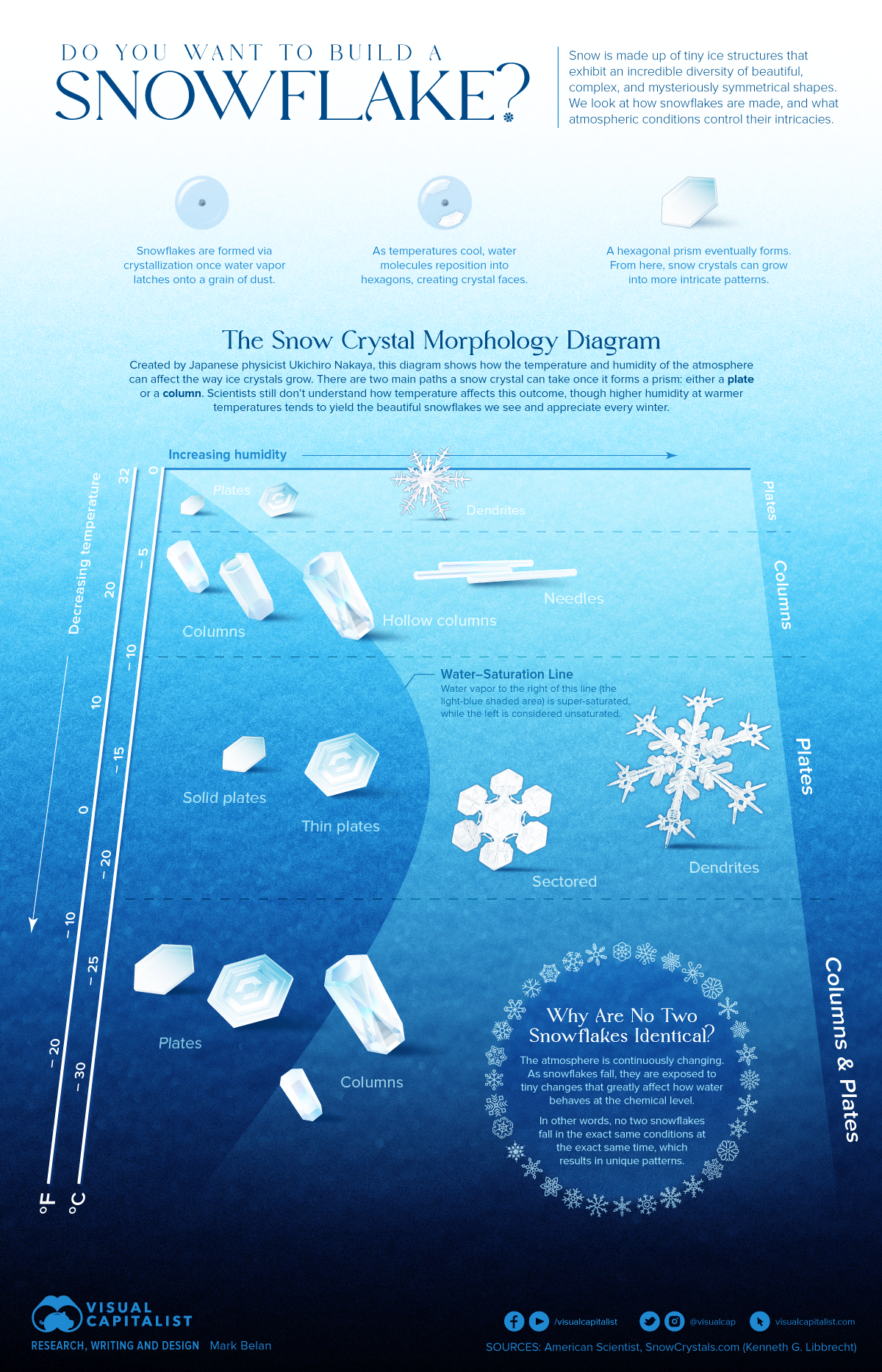
The Nakaya/ Snow Crystal Morphology Diagram of Snowflakes
A Japanese physicist, Ukichiro Nakaya, created the first artificial
snowflakes in the 1930s, and studied their growth as an analog for natural snow
crystal formation. The Snow Crystal Morphology Diagram, or the Nakaya Diagram,
is a chart in the reach that illustrates how snowflakes are formed.
The diagram illustrates the kinds of snowflakes that form
via atmospheric temperature and humidity during a snow crystal’s fall to the
ground.
Factors such as Snowflake size and complexity depend on the
humidity of the atmosphere. The more the water, the larger and more complex the
snowflakes are.
Surprisingly, snowflakes cycle between two classes of growth
(plates vs. columns) as temperatures decrease.
Close to its 100-year anniversary, this detail of the Nakaya
diagram still puzzles researchers today. Many continue to theorize and
demonstrate how this phenomenon may be possible.
Snowflakes Start the Same Journey, but Finish Different
The question that rises here is that, how it is possible
that no two snowflakes are identical if they all have a hexagonal inception and
can form only columns or plates?
The answer lies in the dynamic nature of the atmosphere.
The atmosphere is constantly changing. As each second goes
by, humidity, temperature, wind direction, and a number of other factors
bombard a snow crystal as it falls to the ground.
Snow crystals are sensitive to the smallest of these
changes. Water vapor that is crystallizing responds to different exposures which
in the long run make new patterns.
Ever since no two snowflakes travel in the exact same path
at the exact same time, no two snowflakes can ever look the exact same. Hence, same
start of the journey yet, ending is different for each.
Infographic by: visualcapitalist